Organic Compounds Worksheet Answers. Is one during which the carbon atom with the OH group is hooked up to a few other carbon atoms . An ester (RCOOR′) has an OR′ group hooked up to a carbonyl carbon atom. The applications geared toward decreasing smoking and alcohol use. For example, within the reaction beneath, the alcohol just isn’t symmetrical.
The formation of the acetal or ketal requires the removing of water and known as a dehydration reaction. These reactions require a catalyst or enzyme to trigger them to happen. The reverse response that breaks apart acetal to type the hemiacetal and the alcohol, requires the addition of a water molecule and is called hydrolysis.

The smell of vinegar, for instance, is due to ethanoic acid . The odor of gyms and unwashed socks is basically attributable to butanoic acid, and hexanoic acid is answerable for the robust odor of limburger cheese.
Organic Compounds Scholar
An ether molecule has about the same solubility in water as the alcohol that is isomeric with it. The hydroxyl group is the useful group of the alcohols. The alcohols are represented by the overall formulation ROH.
Displaying all worksheets related to – Naming Of Organic Compounds. Changing the order of Amino acids and adding Amino acids.

Which compound has the upper boiling point—CH3CH2CH2CH2CH2OH or CH3CH2CH2COOH? Which compound has the upper boiling point—CH3CH2CH2OCH2CH3 or CH3CH2CH2COOH?
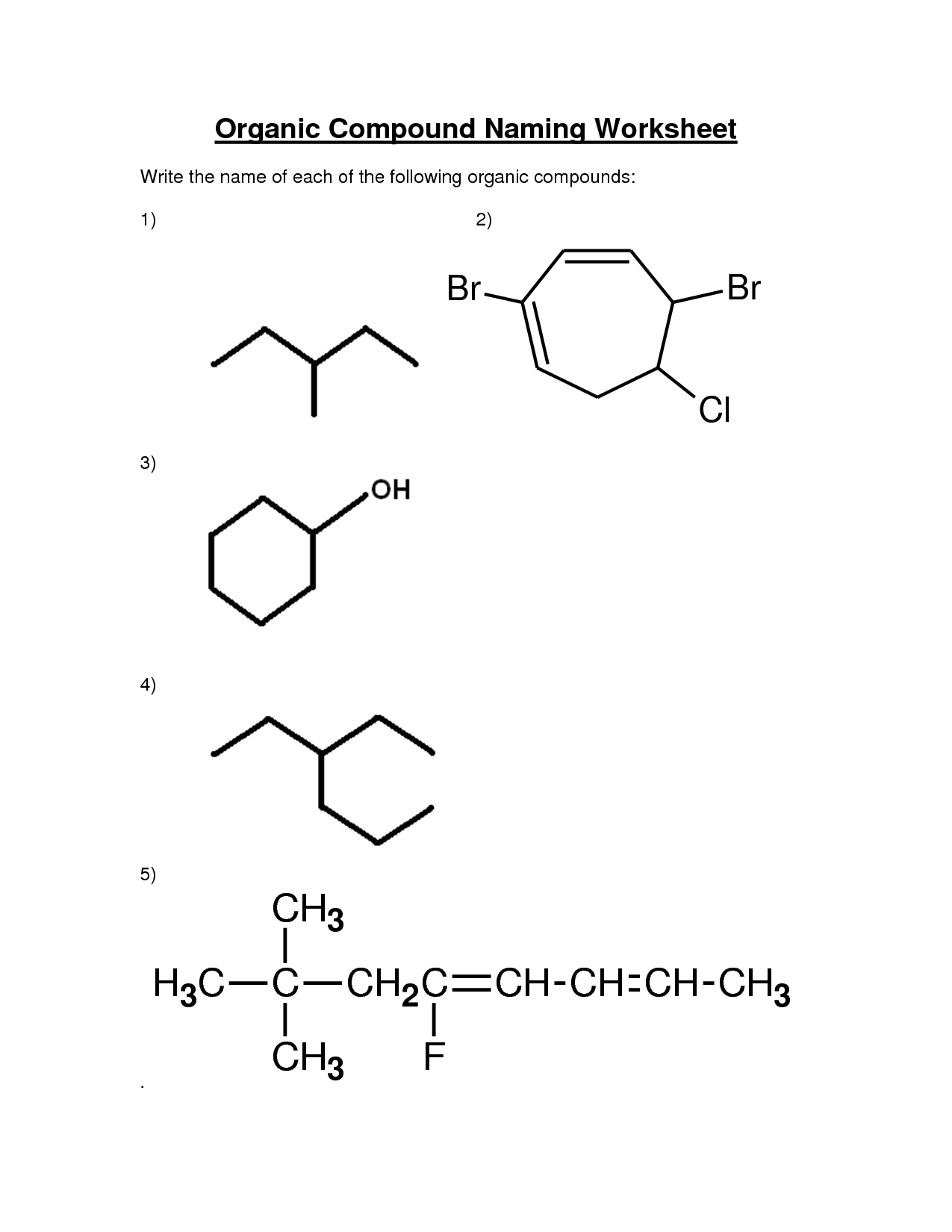
Organic Compounds Worksheet
Esters additionally make up the majority of animal fat and vegetable oils as triglycerides. The formation of lipids and fat shall be described in more detail in Chapter 11.

This alcohol has the OH group on a carbon atom that’s attached to 2 other carbon atoms, so it is a secondary alcohol; oxidation offers a ketone. Carboxylic acids are natural compounds which incorporate a carboxyl practical group, CO2H.
Hydrocarbons & Properties Of Organic Compounds: 12
Thus, ethers containing up to three carbon atoms are soluble in water, due to the formation of H-bonds with water molecules. Esters are pleasant-smelling compounds which may be liable for the fragrances of flowers and fruits. They have lower boiling factors than comparable carboxylic acids as a end result of, even though ester molecules are considerably polar, they can not interact in hydrogen bonding.

Use numbers, commas, dashes, and spaces the place acceptable.To review aldehyde nomenclature and see some examples, click on the button under. State the proper IUPAC name of the following ketones. Use numbers, commas, dashes, and spaces the place appropriate.To evaluate ketone nomenclature and see some examples, click the button beneath.
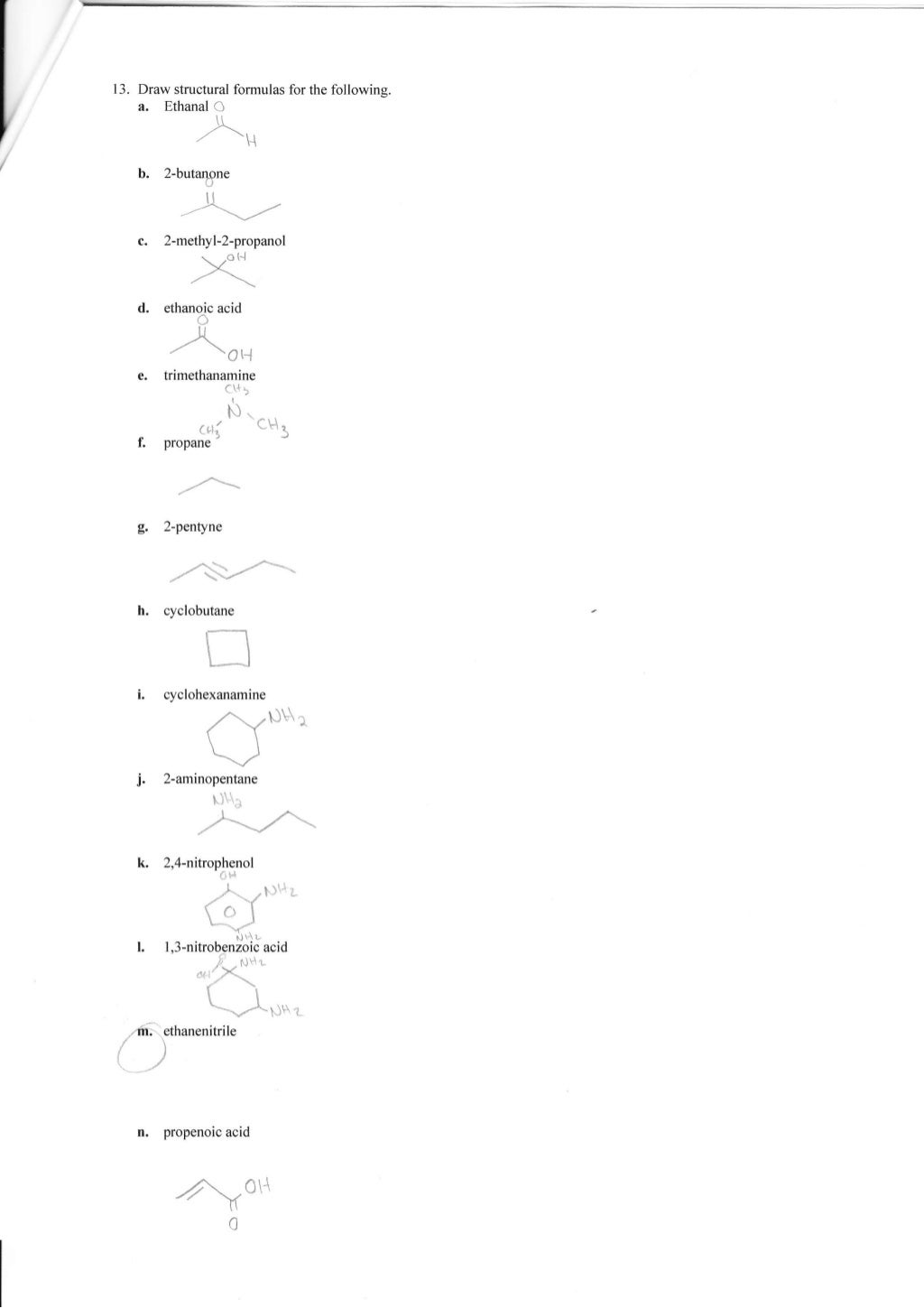
Natural Compounds Worksheet
They have similar lengths, and similar numbers of electrons. These attractions get stronger because the molecules get longer and have extra electrons.
Elimination reactions that occur with more complex molecules may end up in a couple of potential product. In these cases, the alkene will type on the extra substituted position . For example, within the reaction beneath, the alcohol just isn’t symmetrical.
In this fashion, we focus consideration on the organic beginning materials and product, quite than on balancing difficult equations. In the mid 1800’s and early 1900’s diethyl ether was used as an anesthetic during surgery, largely changing chloroform as a result of lowered toxicity.

A general anesthetic acts on the mind to provide unconsciousness and a basic insensitivity to feeling or pain. Diethyl ether was the first basic anesthetic to be commonly used.
Jobs In Assam
IN coordinate covalent bond electrons that are shared by both the atoms are comtributed by only one atom . Cursive handwriting worksheets free apply worksheets for uppercase and lowercase letters a to z.

Anti fragrant compounds follows 4npi electron rule and are cyclic compounds, planar, where as non fragrant are non planar or planar, cyclic or acyclic. DDT, Gammaxene had been used however have been scraped out as they pose health hazards and are insects are ready maintain the max. Generally, Semio-chemicals, a class of compounds present in bugs themselves are being studied and used as effective insecticides and warfare brokers.
- Diethyl ether was the first general anesthetic to be commonly used.
- As a specific instance of an esterification response, butyl acetate may be created from acetic acid and 1-butanol.
- Write an equation for the oxidation of every alcohol.
- Temperature, pH, structure of compound i.e., whether or not the compd is straight chain or branched & molecular weight of compd.
- 28 Naming Organic Compounds Worksheet Organic Chemistry Organic Chemistry Study Chem…
Further condensation reactions then happen, producing polyester polymers. Carboxylic acids are named such as a outcome of they will donate a hydrogen to supply a carboxylate ion. The components which have an result on the acidity of carboxylic acids might be discussed later.

Which public health measure has decreased the incidence of spina bifida? The programs geared toward reducing smoking and alcohol use.

Thus, there are two attainable products of the elimination reaction, option 1 and option 2. Thus, choice 1 would be the major product of the reaction and possibility 2 would be the minor product.

Alcohols can also engage in hydrogen bonding with water molecules, and people with as a lot as about 4 carbon atoms are soluble in water. Removal of the hydrogens and their electrons leads to the formation of a carbonyl functional group. In the case of a main alcohol, the result is the formation of an aldehyde.
Propionic acid reacts with NaOH to type sodium propionate and water. Propionic acid ionizes in water to type a propionate ion and a hydronium (H3O+) ion.

Use numbers, commas, dashes, and areas in your names wherever acceptable.The syntax required could be very particular. To evaluation hydrocarbon nomenclature and see some examples, click the button below.

A constructive cost that is on an electronegative atom. Interested in seeing how well you understand a particular chemistry concept?

Use above the arrow to indicate an oxidizing agent. If no response occurs, write “no reaction” after the arrow. The acetaldehyde is in flip oxidized to acetic acid , a traditional constituent of cells, which is then oxidized to carbon dioxide and water.

Ad Download over K-8 worksheets overlaying math studying social studies and extra. View Nomenclature of Organic Compounds Worksheetdocx from BIOLOGI 78 at Ontario High School Ontario.

Notice that these all have precisely the identical finish to the molecule. All that differs is the complexity of the opposite carbon group attached.
Carbon has 4 valence electrons , and thus, forms four bonds. Nitrogen has 5 valence electrons , and thus, varieties three bonds.

Phenols are compounds having the OH group connected to an aromatic ring. Many alcohols can be synthesized by the hydration of alkenes. Common alcohols embrace methanol, ethanol, and isopropyl alcohol.
That implies that the boiling points shall be greater than these of equally sized hydrocarbons – which solely have dispersion forces. It is fascinating to compare three equally sized molecules.

Oxygen has six valence electrons , and thus, forms two bonds. Halogens have seven valence electrons , and thus, kind one bond.
Oxidation forms first an aldehyde and further oxidation varieties a carboxylic acid. In chapter 8, we learned that alcohols may be shaped from the hydration of alkenes throughout addition reactions.

As the carbon chain increases in length, solubility in water decreases. The borderline of solubility happens at about 4 carbon atoms per oxygen atom. All aldehydes and ketones are soluble in organic solvents and, normally, are much less dense than water.

Ethers (ROR′, ROAr, ArOAr) are compounds during which an oxygen atom is joined to 2 natural teams. Ether molecules haven’t any OH group and thus no intermolecular hydrogen bonding.

Quiz ppt has been included on alcohols and carboxylic acids; the answers to the worksheets have additionally been included to assist college students examine appropriately. As shown above within the alcohol part, aldehydes can endure oxidation to provide a coarboxylic acid.
This is as a result of molecules could have a couple of kind of intermolecular interactions. In addition to hydrogen bonding, alcohol molecules even have LDFs that happen between the nonpolar portions of the molecules. As we saw with the alkanes, the larger the carbon chain, the extra LDFs that are current throughout the molecule.
[ssba-buttons]